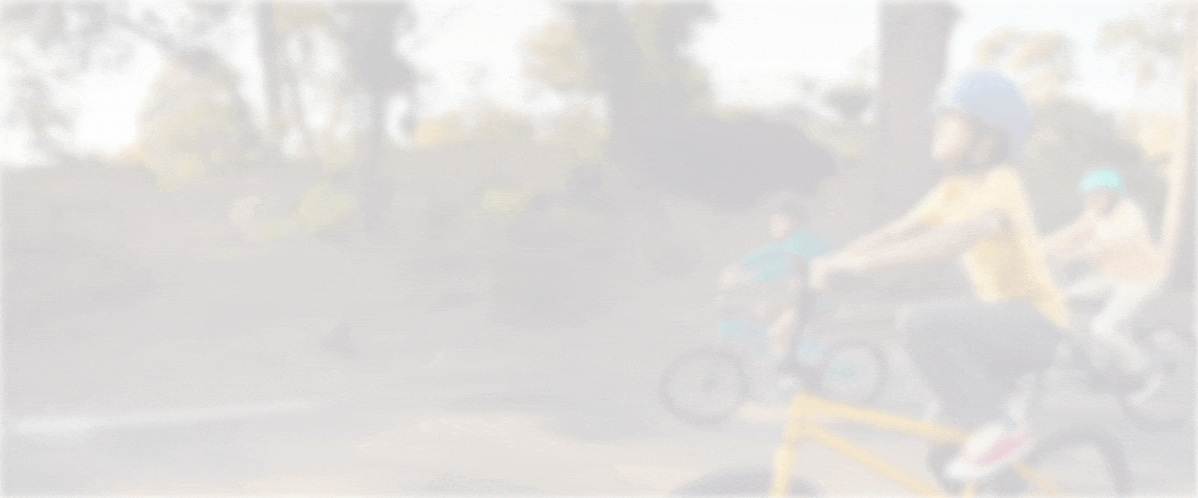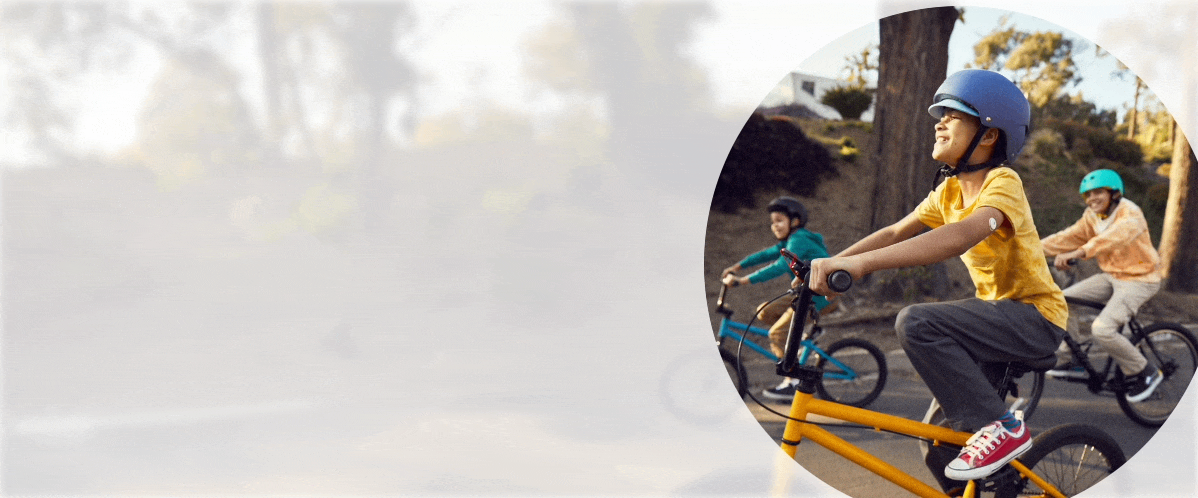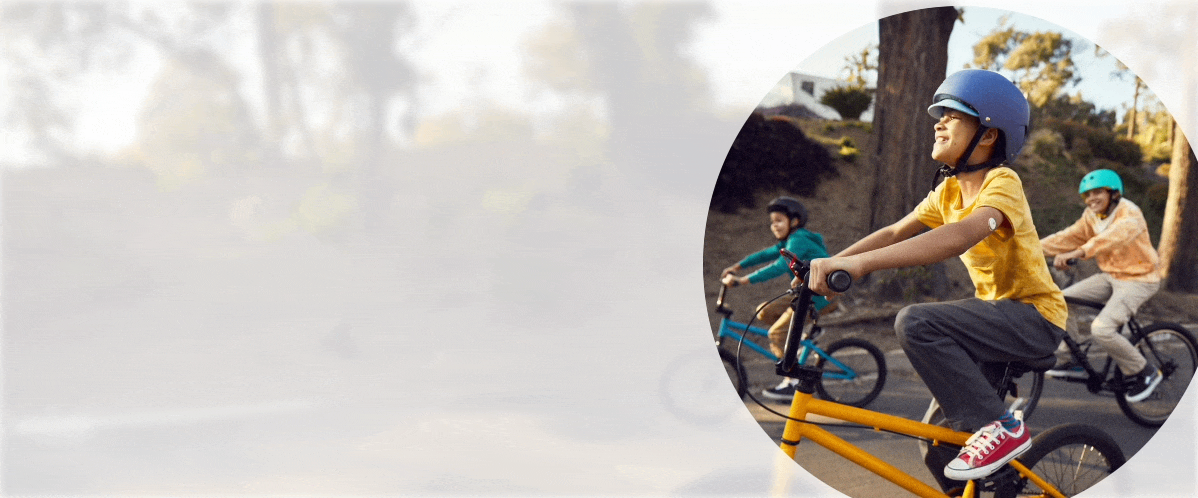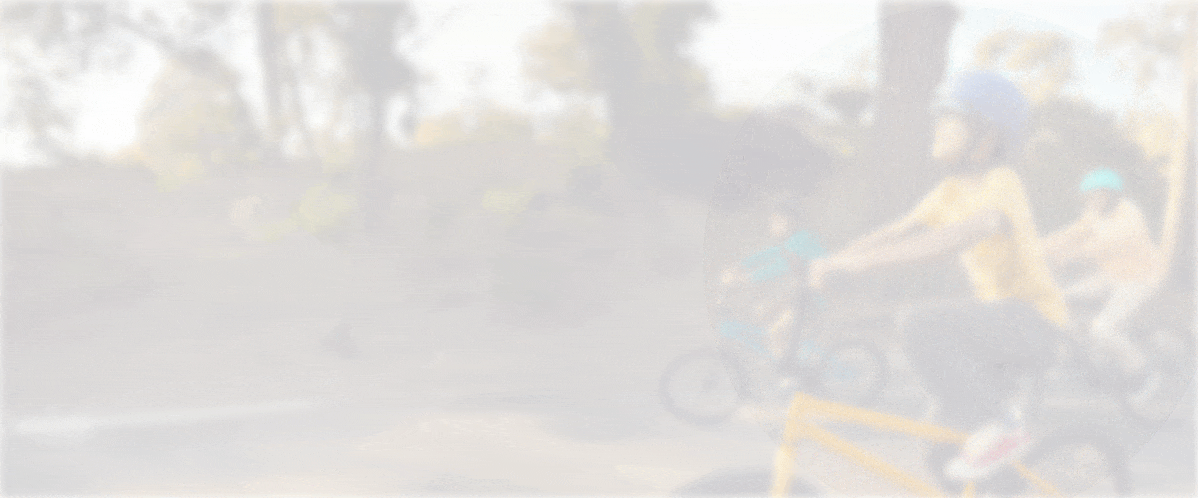
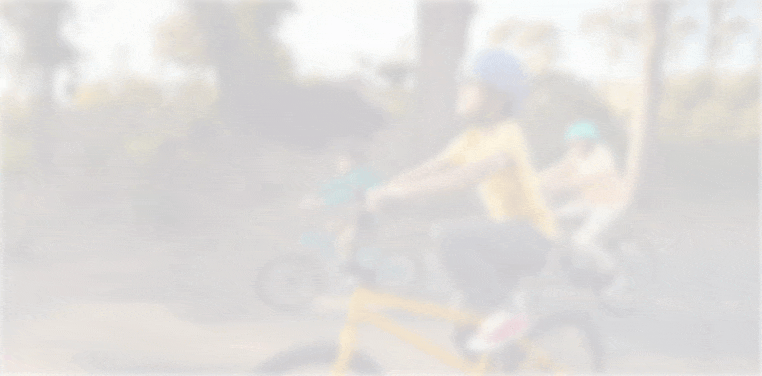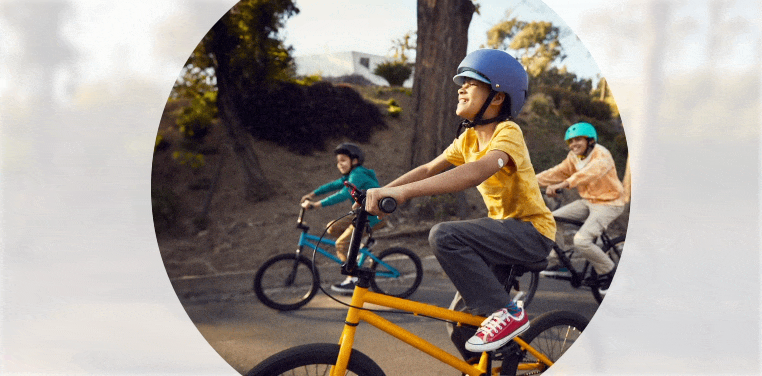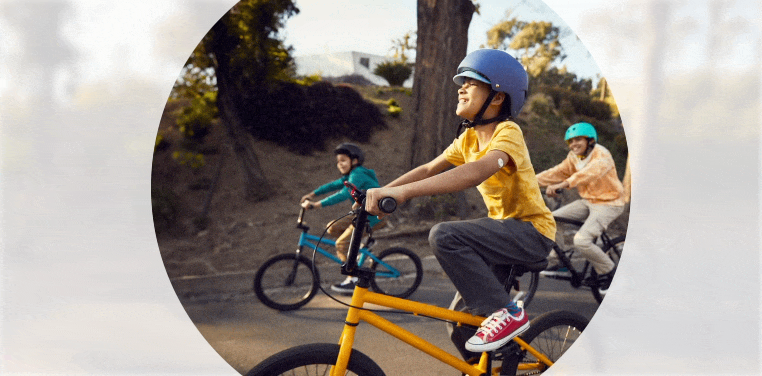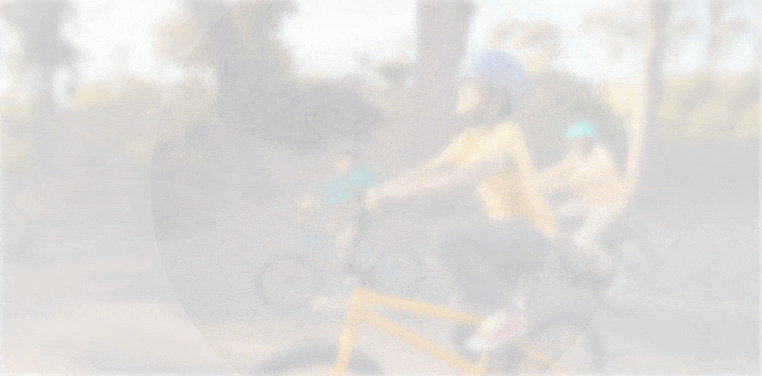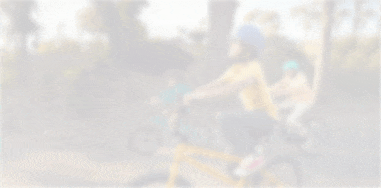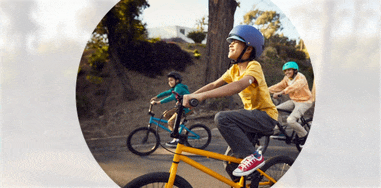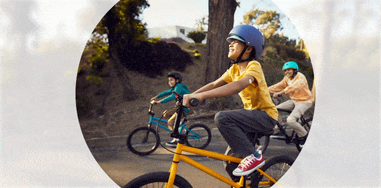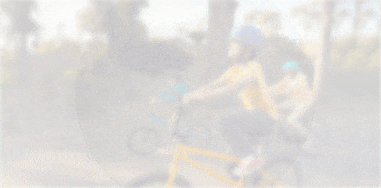Quick and painless1,‡2 application with a sensor that is easy to apply1 and wear1,‡2.
Free to play ball
With the FreeStyle Libre 3 and FreeStyle Libre 2 systems, your child no longer needs frequent fingersticks.
Your child is free to be a kid when wearing a small, discreet sensor on the back of their arm—no fingersticks* needed.
You and your child will both have accurate3,4, minute-to-minute readings so you can act faster to avoid a hypo.
Prepare your child for future self-management with optional alarms§ that help you know when to take action.
Seamlessly share glucose readings with other caregivers||¶ and with healthcare providers¶#.
Discover the FreeStyle Libre 3 system—consistent and accurate4, all the time**.
Accurate
Proven accuracy4 for the most important glucose decisions.
Discreet
The world’s smallest, thinnest††, and most discreet5 sensor—smaller than two stacked pennies.
Next-gen
Longest data storage‡‡6—15-day data storage makes data gaps a thing of the past.
Manage diabetes together with our apps.
The Libre app§§
Working with your child’s sensor, the Libre app makes it easier|||| to stay on top of their glucose with automatic updates on your smartphone every minute. There is an option to silence select alarms§¶¶ for up to 6 hours to experience fewer interruptions throughout the day.
LibreLinkUp app||
The LibreLinkUp app allows for sharing¶ of glucose readings with loved ones and caregivers. Readings are sent from the Libre app to the LibreLinkUp app on the caregiver’s smartphone when sharing is enabled, so children don’t have to monitor their glucose alone.
Try the FreeStyle Libre 3 system for FREE†!
When you sign up for the MyFreeStyle program, your child may be eligible for a free sensor if you're privately insured or cash paying. MyFreeStyle provides guided continuous glucose monitoring (CGM) support for you and your child, so you can manage their diabetes with confidence.
Ready to get started?
Discover the book series written just for them!
Empowering all children, one page at a time.
Download the series for free! Each story takes your child on an exciting adventure designed to educate young audiences and build confidence. Read these inspiring stories together, and share them with friends and family.
Billy and the Bionic Badge
Billy learns how to stay active while wearing a CGM device!
Riley and the Magic Range
Riley discovers that a low can make her magic misbehave!
Marina and the Microscope
Marina invents a microscope to learn how diabetes affects her body!
![]()
The more affordable CGM system***5
More people pay $0 for FreeStyle Libre systems than any other CGMs†††5.
Learn more
The FreeStyle Libre 3 system and FreeStyle Libre 2 system are indicated for use in people with diabetes age 4 and older.
FreeStyle Libre 2 Plus sensor and 3 Plus sensor are indicated for use in people with diabetes age 2 and older.
Medicare and other payor criteria may apply. Abbott provides this information as a courtesy and does not guarantee payment or coverage.
* Fingersticks are required if your glucose alarms and readings do not match symptoms or when you see Check Blood Glucose symbol during the first twelve hours.
† or ♢ Eligible patients will receive one (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor for users with a compatible mobile phone operating system at $0 copay. The expiration date of the voucher is 60 days from the issue date. This program is available for patients with Type 1 diabetes or Type 2 diabetes or gestational diabetes. Patients ages 18 and older are eligible to sign up and receive an offer for the (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor. Patients ages 2–17 are eligible to receive an offer for the (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor through their parent or guardian. This offer is void where prohibited by law. Abbott may modify or rescind this offer at any time without notice. The discounts are not available to beneficiaries of Kaiser Permanente, Medicare, Medicaid or other federal or state healthcare programs, residents of Massachusetts, or US territories (other than Puerto Rico). The free (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor is provided as a sample and is limited to one sample per eligible person per product identification number. The FreeStyle Libre 2 Plus sensor or FreeStyle Libre 3 Plus sensor cannot be re-sold, traded nor submitted to any third-party payer for reimbursement and is not provided as any inducement for future purchases. The free sample card is not health insurance.
‡ Study was performed with the outside US version of the FreeStyle Libre 14 day system. Data is applicable to FreeStyle Libre 2 and FreeStyle Libre 3 systems, as feature sets are similar as FreeStyle Libre 14 day system, excluding alarms.
§ Alarm notifications will only be received when alarms settings are enabled and turned on and sensor is within 20 feet (FreeStyle Libre 2) or 33 feet (FreeStyle Libre 3) unobstructed of the reading device.
|| The LibreLinkUp app is only compatible with certain mobile devices and operating systems. Please check www.librelinkup.com for more information about device compatibility before using the app. Use of the LibreLinkUp app requires registration with LibreView. LibreLinkUp is not intended to be used for dosing decisions. The user should follow instructions on the continuous glucose monitoring system. LibreLinkUp is not intended to replace self-monitoring practices as advised by a physician.
¶ The user's device must have internet connectivity for glucose data to automatically upload to LibreView and to transfer to connected LibreLinkUp app users.
# The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose meter data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
** 60-minute warm-up required when starting the sensor.
†† Among patient-applied sensors.
‡‡ In comparison with Dexcom G7 and Medtronic Guardian Connect System User Guide.
§§ The FreeStyle Libre systems apps are only compatible with certain mobile devices and operating systems. Please check the Support section of our website for more information about device compatibility before using the apps. Use of the FreeStyle Libre systems apps may require registration with LibreView.
|||| The Libre App works with Libre 2, Libre 2 Plus, Libre 3 and Libre 3 Plus sensors and glucose data updates automatically without scanning the sensor.
¶¶ Silent mode allows users to silence their signal loss and glucose alarms, including urgent low glucose alarm for up to six hours. You will not hear your glucose and signal loss alarms even if you've turned on Override Do Not Disturb but may still get the visual and vibratory notifications based on your phone's settings.
## Study was performed with the US version of the FreeStyle Libre 14 day system. Data is applicable to the FreeStyle Libre 2 and FreeStyle Libre 3 systems, as feature sets are similar as FreeStyle Libre 14 day system, excluding alarms.
*** Based on a comparison of list prices of the FreeStyle Libre personal CGM systems versus competitors’ CGM systems, assuming annual use of one receiver (or equivalent hardware) and quantity of transmitters and/or sensors according to use life. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
††† Based on prescription claims for the aggregate of patients covered by Commercial insurance, Managed Medicare, Managed Medicaid using the FreeStyle Libre personal CGM systems versus competitors’ CGM systems. Does not include fee-for-service Medicare, fee-for-service Medicaid, and uninsured patients. The actual amount a patient pays may vary. The FreeStyle Libre personal CGM systems require a prescription.
References: 1. Alva, Shridhara, et al. “Accuracy of the Third Generation of a 14-Day Continuous Glucose Monitoring System.” Diabetes Therapy 14, no. 4 (2023): 767–776. https://doi.org/10.1007/s13300-023-01385-6. 2. Campbell, Fiona M., et al. "Outcomes of using flash glucose monitoring technology by children and young people with Type 1 diabetes in a single arm study." Pediatric Diabetes 19, no. 7 (2018): 1294–1301. https://doi.org/10.1111/pedi.12735. 3. FreeStyle Libre 2 User's Manual. 4. FreeStyle Libre 3 User's Manual. 5. Data on file. Abbott Diabetes Care, Inc. 6. Based on FreeStyle Libre 3 User's Manual, Dexcom G7 CGM User Guide and Medtronic Guardian Connect System User Guide. 7. Unger, Jeff, Pamela Kushner, and John E. Anderson. “Practical Guidance for Using the FreeStyle Libre Flash Continuous Glucose Monitoring in Primary Care.” Postgraduate Medicine 132, no. 4 (2020): 305–313. https://doi.org/10.1080/00325481.2020.1744393.
ADC-33111 v8.0