AID systems + CGM sensors:
What you need to know.
What you need to know.
Automated insulin delivery (AID) systems include an insulin pump and a continuous glucose monitor (CGM) that work together to automatically deliver insulin in response to glucose trends.
When connected to a CGM sensor like the FreeStyle Libre 3 Plus sensor or FreeStyle Libre 2 Plus sensor, compatible AID systems are designed to precisely adjust your insulin as your glucose goes up and down. Explore the products below and find a system that works for you.
Explore compatible AID systems




Sensor |
Partner |
AID (Automated Insulin Delivery) System |
|---|---|---|
 
|
|
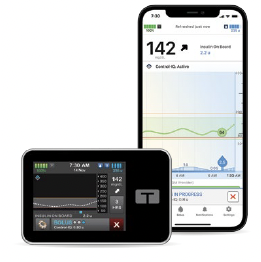
t:slim X2™ insulin pumpt:slim mobile app
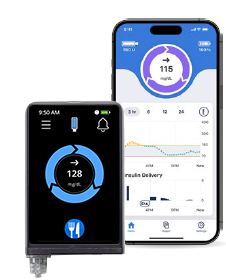
iLet Bionic PancreasiLet mobile app
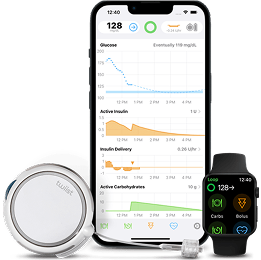
twiist™ Automated Insulin Delivery Systemtwiist app |
 
|
|
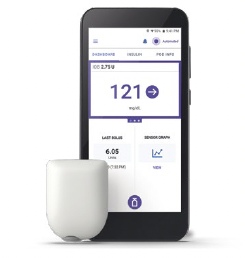
Omnipod® 5 Automated Insulin Delivery SystemOmnipod 5 app† |
![]()
The t:slim X2 insulin pump is powered by Control-IQ+ technology, which uses FreeStyle Libre 3 Plus sensor values to predict glucose levels 30 minutes ahead and automatically adjust insulin as needed. Only Tandem pumps have AutoBolus, which helps with missed meal boluses and preventing hyperglycemia.
![]()
The Beta Bionics iLet Bionic Pancreas communicates with the FreeStyle Libre 3 Plus sensor to personalize insulin doses by making adjustments as you need them.
iLet users can now share their glucose and insulin data with up to 10 family, friends, and caregivers using the Bionic Circle app.

The twiist AID System powered by Tidepool and the FreeStyle Libre 3 Plus sensor create one easy*, integrated experience. Automatic readings are sent straight from the sensor to the twiist pump every minute.
The twiist insiight app allows parents and caregivers to see the glucose and insulin data of their loved ones.

Omnipod 5 is a tubeless, waterproof§ automated insulin delivery system that works with the FreeStyle Libre 2 Plus sensor. SmartAdjustTM technology proactively predicts glucose values 60 minutes into the future to correct highs and to help to protect against lows, day and night1.
You can shareII your glucose data with family, friends, and caregivers using the LibreLinkUp app¶.
Caregivers can set and receive glucose alarmsII,# and notifications in the LibreLinkUp app.
Need additional support?
Tandem Customer Service can be reached at 1-877-801-6901.
Ask your doctor about the FreeStyle Libre 3 Plus sensor and t:slim X2 insulin pump.
Beta Bionics Customer Service can be reached at 1-855-745-3800.
Ask your doctor about the FreeStyle Libre 3 Plus sensor and iLet Bionic Pancreas insulin pump.
Sequel Customer Service can be reached at 1-877-489-4478.
Ask your doctor about the FreeStyle Libre 3 Plus sensor and the twiist AID System.
Insulet Customer Service can be reached at 1-800-591-3455.
Ask your doctor about the FreeStyle Libre 2 Plus sensor and the Omnipod 5 AID System.
The FreeStyle Libre 3 system includes the Libre 3 Plus sensor, Libre 3 sensor, Libre 3 app, Libre App, and the Libre 3 reader.
The FreeStyle Libre 2 system includes the Libre 2 Plus sensor, Libre 2 sensor, Libre 2 app, Libre App, and the Libre 2 reader.
FreeStyle Libre 2 Plus sensor and 3 Plus sensor are indicated for use in people with diabetes age 2 and older.
* Based on sensor features including up to 15-day wear period, automatic readings every minute, accuracy data, and single-app setup with AID systems.
† The Omnipod 5 App is available in the dedicated Controller.
§ The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Personal Diabetes Manager and Controller are not waterproof.
II The user’s device must have internet connectivity for glucose data to automatically upload to LibreView and to transfer to connected LibreLinkUp app users.
¶ Check the Support section of www.librelinkup.com for information about mobile device and operating system compatibility. LibreLinkUp is not intended to be used for dosing decisions or to replace self-monitoring practices as advised by a physician and requires registration with LibreView.
# In order to receive alarms the FreeStyle Libre 2 Plus sensor must be connected to the Omnipod 5 System.
♢ Eligible patients will receive one (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor for users with a compatible mobile phone operating system at $0 copay. The expiration date of the voucher is 60 days from the issue date. This program is available for patients with Type 1 diabetes or Type 2 diabetes or gestational diabetes. Patients ages 18 and older are eligible to sign up and receive an offer for the (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor. Patients ages 2–17 are eligible to receive an offer for the (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor through their parent or guardian. This offer is void where prohibited by law. Abbott may modify or rescind this offer at any time without notice. The discounts are not available to beneficiaries of Kaiser Permanente, Medicare, Medicaid or other federal or state healthcare programs, residents of Massachusetts, or US territories (other than Puerto Rico). The free (1) FreeStyle Libre 2 Plus sensor or (1) FreeStyle Libre 3 Plus sensor is provided as a sample and is limited to one sample per eligible person per product identification number. The FreeStyle Libre 2 Plus sensor or FreeStyle Libre 3 Plus sensor cannot be re-sold, traded nor submitted to any third-party payer for reimbursement and is not provided as any inducement for future purchases. The free sample card is not health insurance.
Reference: 1. Omnipod user manual.
Important Safety Information for Tandem t:slim X2 insulin pump: For full Tandem safety information, please visit www.tandemdiabetes.com/safetyinfo.
Important Safety Information for iLet Bionic Pancreas System: For full Beta Bionics safety information, please visit www.betabionics.com/safety.
Important Safety Information for Sequel twiist AID System: For full twiist AID System safety information, please visit www.twiist.com/safety.
Important Safety Information for Insulet Omnipod 5: For full Omnipod 5 safety information, please visit www.omnipod.com/safety.
The sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott. Other trademarks are the property of their respective owners.
ADC-85449 v7.0